
Friedel craft alkylation reaction
In this Friedel craft alkylation reaction Arenes, an alkyl group is introduced on the benzene or arene ring by using the alkyl halide in presence of anhydrous AlCl3. The reagents can be represented as
- Anhydrous AlCl3
- Anhydrous FeCl3
- BF3
- TiCl3
- ZnCl2
- SnCl4
The mechanism of the reaction starts with the formation of an Alkyl carbonium ion. Alkyl halide reacts with anhydrous AlCl3 and acts as a halogen carrier to produce an alkyl carbonium ion (electrophile). The stability of an alkyl carbonium ion is important.

The alkyl carbonium ion formed reacts with an aromatic ring to produce resonating forms of the arene. This positive charge on the aromatic ring a stabilized by resonating forms.
In resonance hybrid structure the positive charges are distributed over five carbon atoms so relative positive charge density on each carbon atom is very less and thus it is stabilized.
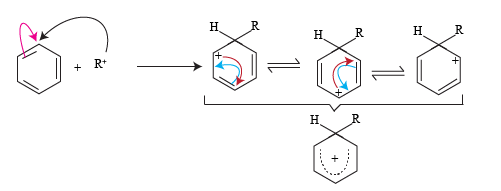
The structure three formed in the resonating forms further stabilizes to give a mono-substituted alkyl derivative.
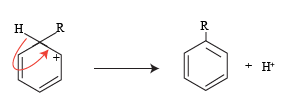
The H+ ion release in this reaction joins with the conjugate base to form a byproduct here it is termed as spent acid.

The monosubstituted derivative of arenes uses an alkyl halide to give the monosubstituted derivative. While deciding the product of alkylation stability of the carbonium ion formed is to be taken into account. As shown in the examples
Check out reactions
| Chlorination Reaction | Bromination Reaction | Nitration Reaction |
| Sulphonation Reaction | Friedel Craft Alkylation Reaction | Friedel craft Acylation Reaction |
You may also like
| Nucleophilic Substitution reaction | Free Radical substitution reaction | Electrophilic addition reaction | Free radical addition reaction |
| Nucleophilic addition reaction | Elimination reaction | Bond Fission |
Our other courses
| Chemistry XII | Mock Test XI | Mock Test XII | |
| Mock Test NEET | Mock Test JEE Mains | Science class 9 |
https://www.myetutors.com/?p=15745&feed_id=86
